Abstract
Immune system effector T cells are a promising potential therapy for cancer treatment. Cellular impedance-based assays are a sensitive, label-free, and nondestructive method of investigating changes in cell physiology and health. In this proof-of-concept study, we investigated the targeting potency of activated human T cells on human U87MG glioma cell monolayers. U87MG growth curves were generated using the Maestro to record real-time, label-free impedance measurement. The addition of activated human T-cells resulted in a decrease of the impedance signal consistent with T-cell-mediated lysis of the glioma cells.
Introduction
Glioblastoma (GBM) is an aggressive form of brain cancer that has no effective treatments and a prognosis of only 12-15 months. Immune system effector T cells hold promise for future cancer therapy due to their high specificity and innate cytotoxicity. Assessing the effectiveness and potency of T cell therapies in vitro is often done via endpoint assays, which are limited to a single snapshot in time. Axion BioSystems’ Maestro Z platform offers impedance-based cell analysis for real-time, label-free monitoring of cells (Figure 1). Continuous data reveals the kinetics of cancer cell response to T cells for better mechanistic understanding without the time- and cost-intensive process of repeating multiple endpoint assays. The Maestro Z impedance assay is a sensitive method to continuously monitor cancer cell proliferation and immune cell-mediated cytotoxicity, providing a powerful means of assessing immune cell potency.
T Cell-Mediated Cytolysis of Glioblastoma using Maestro Z Real-Time Impedance Assay
Materials and Methods
Cells and reagents
U87MG, hereafter referred to as U87 cells, glioblastoma cells (Cat. HTB-14) were obtained from ATCC (Manassas, VA). T cells were obtained from a patient (line H24FW).
Experimental components
Poly-D-lysine, or PDL, (Cat. P6407-5MG) was purchased from Sigma Aldrich (St. Louis, MO). ImmunoCult-XF T Cell Expansion Medium (Cat.10981) and ImmunoCult Human CD3/CD28 T Cell Activation Serum (Cat 1971) were obtained from STEMCELL Technologies (Vancouver, BC). U87 medium consisted of Gibco DMEM/F-12 (Cat. 21331020) supplemented with 10% FBS and 1% penicillin-streptomycin.
CytoView-Z 96 plate
A CytoView-Z 96-well plate (Axion BioSystems) was used for the experiment. The plate is composed of a polyethylene terephthalate (PET) surface with a gold recording electrode embedded in the culture surface of each well. Humidity reservoirs on the plate were filled with deionized water to maintain humidity.
Maestro Z assay platform
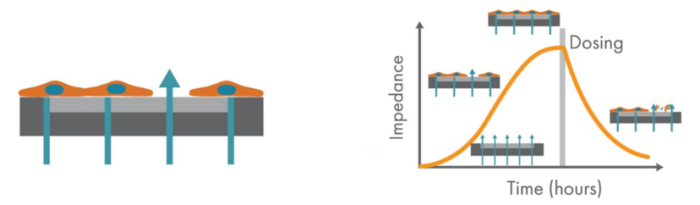
The Maestro Z platform (Axion Biosystems) uses impedance measurements (ohms, Ω) to quantify the presence of cells on the electrode. To measure impedance, small electrical signals are delivered to the electrodes. Cell attachment, spreading, and cell-cell connections block these electrical signals and are detected as an increase in impedance. Impedance is also sensitive to subtle changes in cell conformation, such as those caused by receptor-mediated signaling or cell morphology. Since impedance is non-invasive and label-free, impedance assays can be used to quantify dynamic cellular responses over minutes, hours, and days. In addition, the Maestro Z has built-in environmental control, finely controlling temperature and CO2 throughout the experiment.
Cell plating
To coat the surface of the plate, 100 µL of PDL was added to each well. The surface-coated plate was incubated in 37C and 5% CO2 overnight. The next day, the surface coating was aspirated from the plate, and 100 µL of U87 medium was added to each well.
Media-only recording
The plate was docked in the Maestro Z and a media-only reference was recorded to provide a baseline for the data collected during the rest of the experiment.
Cell culture
U87MG cells were thawed and cultured in accordance with supplier recommendations. Trypsin (0.05%) was used to detach and collect cells from the flasks. A sample of the cell suspension was used to count cells to determine the total number of viable cells using Trypan blue solution. The cell suspension was transferred to a 15 mL conical tube and the cell suspension was pelleted by centrifugation. The supernatant was aspirated, and the cell suspension was diluted in U87 medium to three final working concentrations of 10,000, 25,000, and 50,000 cells per 100 µL. Cells were seeded into the CytoView-Z plate using a 100 µL addition per well (Figure 2). Twelve wells were plated for each of the three cell densities. Twelve additional wells were filled with U87 medium to serve as media reference wells. Sterile DI water (6 mL) was added to the humidity reservoirs on the edges of the plate. The plate was left to rest in the biosafety cabinet for one hour at room temperature.
Impedance recordings
The plate was docked in the Maestro Z and the automatic environment controls set the chamber to 37C and 5% CO2. Impedance measurements were recorded every 15 minutes for 24 hours prior to T cell addition, and for 120 hours after T cell addition.
T cell preparation and addition
T Cells were activated using ImmunoCult CD3/CD28 Activation Serum 24 hours prior to addition to U87 cells and maintained in ImmunoCult-XF T Cell Expansion Medium. After 24 hours, T cells were added in a 10:1 ratio (T cells: U87 cells) to 4 wells per U87 cell density. Media for untreated U87 cell wells and media reference wells was replaced with ImmunoCult-XF T Cell Expansion Medium.
Results
U87 cell proliferation
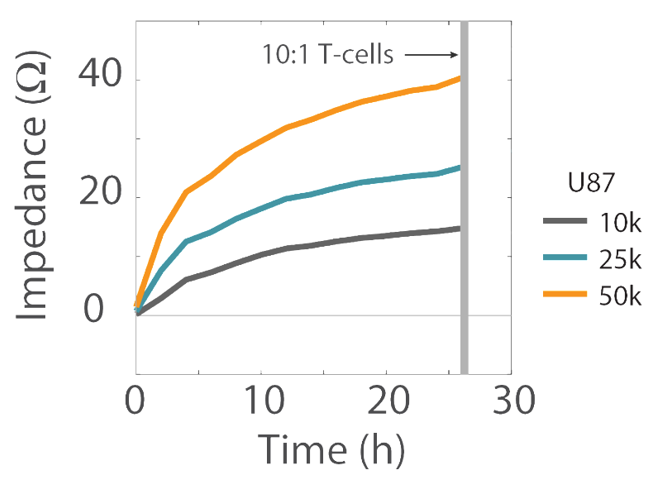
Impedance was tracked continuously for U87 cells plated at the three different densities (10K, 25K, and 50K) using the Maestro Z system (Figure 3). The increase in impedance signal corresponded to the density of cells plated, such that higher cell densities resulted in larger impedance values and proliferation of those higher cell densities leads to increasing impedance values over time.
T cell-mediated cytolysis
The addition of activated human T cells resulted in a decrease in the impedance signal, consistent with T-cell-mediated lysis of U87 cells, while untreated wells showed a continual increase in impedance, consistent with continued cell proliferation (Figure 4).
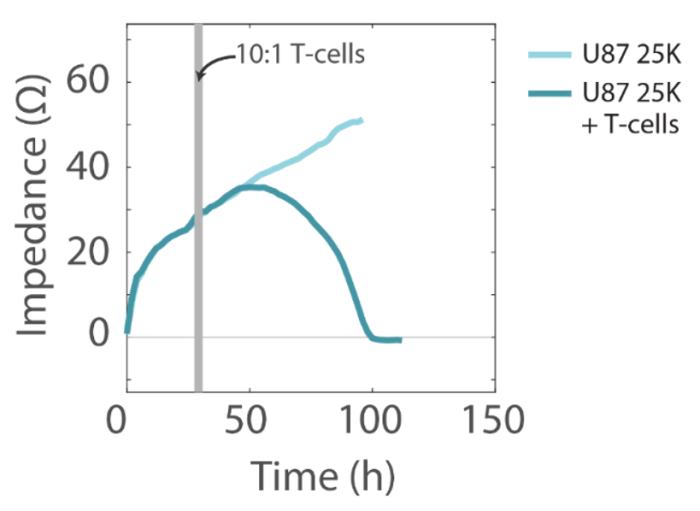
T cell concentration-dependent effects
Overall, higher T cell densities led to a faster killing of U87 cells (Figures 5 and 6). The time required to kill 50% of the U87 cells (KT50) was approximately 35 hours in the wells with 500k T cells, as opposed to approximately 80 hours in the wells with 100k T cells.
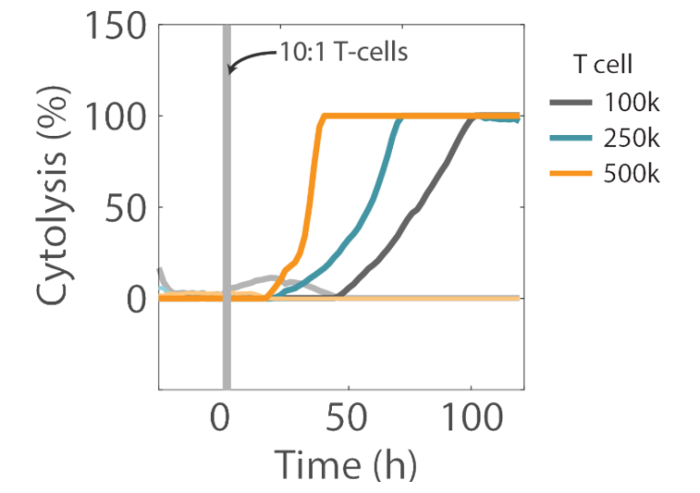
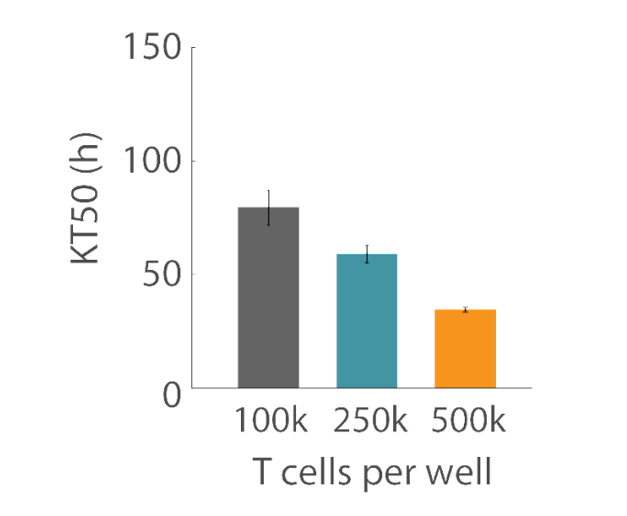
Conclusion
The Maestro Z impedance assay is a complete solution, providing a simple, real-time, label-free method to continuously monitor cancer cell proliferation and immune cell-mediated cytotoxicity.